It freezes at 79 C 178 F and boils at 1635 C 3263 F. Rank from highest to lowest boiling point.
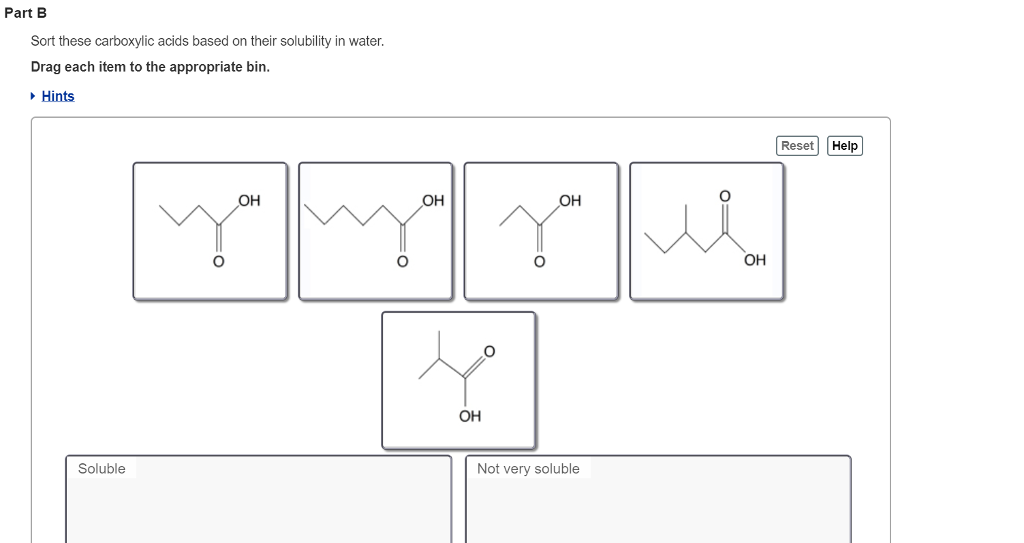
Solved Part B Sort These Carboxylic Acids Based On Their Chegg Com
As the chain length of the carboxylic acids increases the solubility in water decreases rapidly.
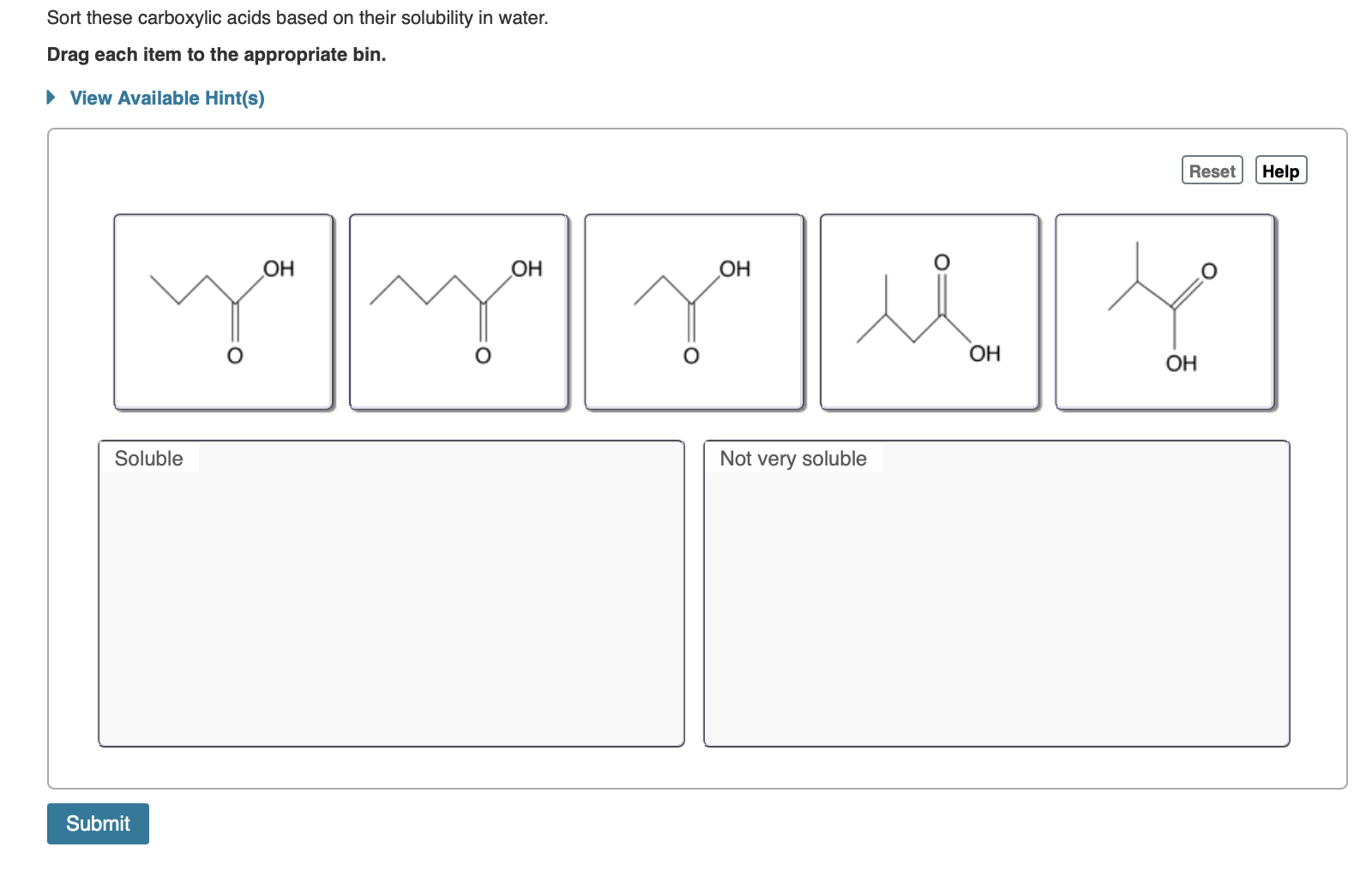
. Palmitic acid CH 3 CH 2 14. Drag each item to the appropriate bin. Sort these carboxylic acids based on their solubility in water.
Yes carboxylic acids are soluble in water because water and carboxylic acids both are polar. Rank them based on their boiling points. Carboxylic acids with five or more carbon atoms are not very soluble because the large alkyl portions diminish the effect of the polar groups.
Hexanoic acid CH 3 CH 2 4 COOH is barely soluble in water about 10 g100 g of water. Previous question Next question. Smaller carboxylic acids 1 to 5 carbons are soluble in water whereas higher carboxylic acids are less soluble due to the increasing hydrophobic nature of the alkyl chain.
Sort these carboxylic acids based on their solubility in water. When you mix the two together the energy released when the new hydrogen bonds form is much the same as is needed to break the hydrogen bonds in the pure liquids. They neutralize basic compounds.
Mg2 Fe3 or Ca2 any of these many sunblock products are labeled as PABA free PABA is the abbreviation for p-aminobenzoic acid. But with the increase of R the solubility of carboxylic acids are decreases this is because of large size of molecule. For unlimited access to Homework Help a Homework subscription is required.
Drag each item to the appropriate bin. However a protic organic solvent such as ethanol can dissolve carboxylic acids containing. Soluble carboxylic acid dissociate to some extent in water to yield hydrogen ions.
As weak acids carboxylic acids will react with strong bases such as and to form a carboxylate anion. Up to 256 cash back What are Esters. Solubility in Hot water.
Those with more than six carbons are slightly soluble in water. Butyric acid is a colourless liquid soluble in water and miscible with common organic solvents. Experts are tested by Chegg as specialists in their subject area.
The carboxylic acids with up to four carbon atoms will mix with water in any proportion. To rank items as. Rank from highest to lowest boiling point.
Note that carboxylic acids of greater than 6 carbon atoms are minimally 1 g100 mL soluble to insoluble in water. Rank from highest to lowest boiling point. Sort these carboxylic acids based on their solubility in water.
The pH of solutions of carboxylic acids is. Up to 256 cash back Sort these carboxylic acids based on theirsolubility in water. Question Sources of Nitric Add in the Atmosphere The reaction between N2O5 and water is a source of nitric acid in the atmosphere.
To rank items as equivalent overlap them. 100 43 ratings Transcribed image text. Rank from highest to lowest boiling pointTo rank items as equivalent overlap them.
It is because of these interactions that carboxylic acids can dissolve in water to form acidic solutions. Correct If the total number of carbon atoms is four or less the. Write equations for the reactions of the carboxylic acids with NaOH.
View Available Hint s. Part C propanoic acidstrong base Draw the resulting carboxylate anion that forms when propanoic acid reacts with a strong base in expanded structural formula including all hydrogens. It is also important to appreciate the solubility of carboxylic acids in solvents other than water.
Write equations for the reactions of the salts of the carboxylic acids with HCl. Acetic acid vinegarSucrose sugarMonosodium glutamate vetsinCellulosecotton fiberIsopropyl or ethyl alcoholFatty acid salts soapKeroseneNaphthalene balls NOTE. The animation below shows the interaction between ethanoic acid and water.
Write the rate law for the reaction. Consider the data provided in the table above for acid solubility in ethanol. Determine what makes a carboxylic acid soluble in water Select the statement below that correctly defines what makes a carboxylic acid soluble in water.
Sort these carboxylic acids based on their solubility in water. These longer chain acids tend to be rather soluble in. Part B Sort these carboxylic acids based on their solubility in water.
To rank items as. Sort these carboxylic acids based on their solubility in water. Solubility decreases as the carbon chain length increases because dipole forces become less important and dispersion forces become more predominant.
The solubility of the bigger acids decreases very rapidly with size. These are the solute to be dissoolve in water and vegetable oil. Benzoic acid was not soluble in water soluble in 5 NaOH and therefore is an A compound.
Sort these carboxylic acids based on their solubility in water. View Available Hint s Reset Help OH OH OH you OH OH Soluble. Butyric acid is manufactured by catalyzed air oxidation of butanal butyraldehyde.
Solubility in Cold water. The pH reading was about 80. When N2O5 is 0132 mM and H2O 230 mM the rate of.
Sodium acetate is classified as an S 1 w compound because it is soluble in water and insoluble in ether. View the full answer. Hints Reset Help OH OH OH Soluble Not very soluble.
Carboxylic acid salts are named in the same manner as inorganic salts. Carboxylic acids and Their Salts Acetic Acid Benzoie Acid A1 Condensed Structural Formulas A2. We review their content and use your feedback to keep the quality high.
When compared with the solubility in water of other compounds of comparable molecular weightwhich of the following is true of their solubility in water of carboxylic acids. It is an alkali metal carboxylate. So this 10 will be mixed with water and vegetable oil Question.
Propanoic acid butanoic acid Isobutanoic acids are soluble in water 1 to 5. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water. With solutions of carbonate CO 3 and bicarbonate HCO 3 ions they also form carbon dioxide gas.
Carboxylic acids with four or fewer carbon atoms are soluble in water and undergo ionization. As with melting and boiling points we notice a similar trend with solubility in water in that carboxylic acids are more soluble in water than their corresponding alcohols. The acids with one to four carbon atoms are completely miscible with water.
A compounds are higher molecular weight carboxylic acids their anhydrides and acyl. The name of the cation is followed by the name of the organic anion. In these reactions the carboxylic acids act like inorganic acids.
Since they are both so small methanoic acid and methanol are infinitely soluble extremely soluble so it is difficult to notice any differences. The reaction is first order in each reactant. Drag each item to the appropriate bin.
We also know the prunciple of solubility ie like dissolve like. Rank from highest to lowest boiling point. Part A The following compounds have approximately the same molar mass.
The carboxylic acids with low molar mass up to four carbon atoms are freely soluble in water.
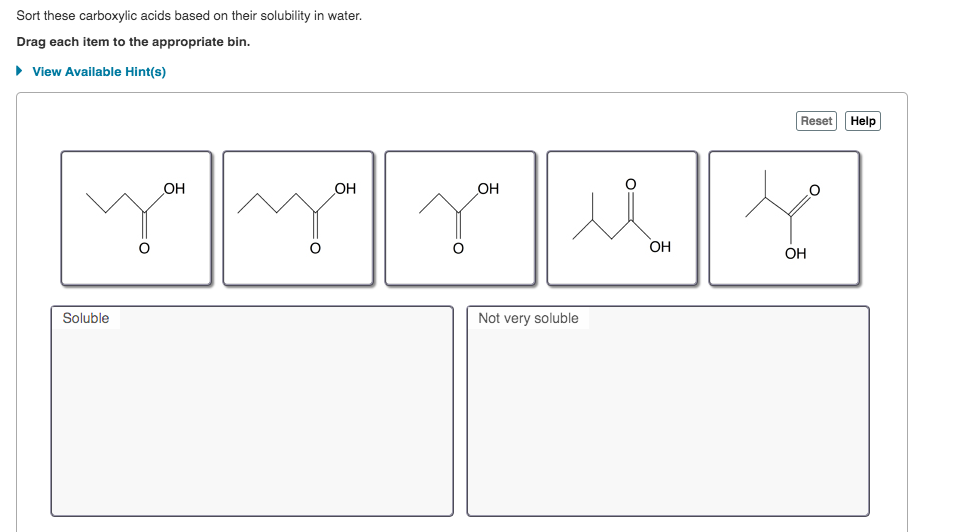
Answered Sort These Carboxylic Acids Based On Bartleby

Solved Sort These Carboxylic Acids Based On Their Solubility Chegg Com

Oneclass Sort These Carboxylic Acids Based On Theirsolubility In Water Rank From Highest To Lowest
0 Comments